Addressing technical barriers to trade is a key priority for the Association of Southeast Asian Nations (ASEAN) as part of trade facilitation in achieving the single market and production base under the ASEAN Economic Community directive agreed in 2015. The region has been undertaking positive steps toward standard harmonization in ASEAN priority sectors, integrating and bringing about regulatory convergence by taking into account the diversities that exist in the ten ASEAN member states.
Health supplements in ASEAN are under the responsibility of the Traditional Medicine and Health Supplement Product Working Group (TMHS PWG). One of the TMHS PWG’s outputs is the ASEAN Agreement on a Regulatory Framework for Health Supplements, which includes various technical requirements for health supplements that participating member states must adopt by adjusting their domestic regulations.
The key ingredients of these health supplements are vitamins and minerals. While all ASEAN countries allow the use of vitamins and minerals in health supplements, the amounts allowed for use vary, depending on the nutritional requirements deemed appropriate by each country.
Partly in response to these efforts for regional harmonization, there have been important changes to vitamin and mineral limits in certain ASEAN countries that are worth monitoring. Some of these are outlined below.
Thailand
In January 2024, the Thai Food and Drug Administration (TFDA) updated the Thai Recommended Daily Intake (Thai RDI) guidelines for the Thai population. Subsequently, the TFDA adjusted the vitamin and mineral limits in order to comply with the updated Thai RDI and to follow the ASEAN Agreement on a Regulatory Framework for Health Supplements.
The TFDA’s adjustment of vitamin and mineral limits for use in food supplements came in Notification of the Ministry of Public Health (No. 448) B.E. 2566 (2023) Re: Food Supplements (No. 5), which was published in the Government Gazette on January 4, 2024. It increased the maximum limits for certain vitamins and minerals (see breakdown below) up to the limits that had previously only been allowed in drugs. The minimum limits of vitamins and minerals for use in food supplements should be more than 15% of Thai RDIs, as set out in the Notification of the Ministry of Public Health (No. 445) B.E. 2566 (2023) Re: Nutrition Labels. This notification took effect on July 2, 2024.
Before that date, if a party wished to use more than 60 mg of Vitamin C or more than 2 mcg of Vitamin B12 in a health supplement, they would have needed to register the product as a medicine (not a health supplement) in Thailand. Now, health supplements are allowed to use 1,000 mg of vitamin C and 600 mcg of vitamin B12—a level that previously would have required registration as a medicine.
Health supplements are classified as a type of food in Thailand—this differs from some other countries, which may include health supplements in the medicine group. The registration process for health supplements is straightforward and takes a short time (about one to two months) to receive approval. On the other hand, drug registration is more complex and time-consuming. Health supplement companies widely agree that the time needed to receive license approval is one of the main obstacles for a business getting their products to market.
This change allows products that in the past had to be registered as medicines to now be accepted as health supplements in Thailand. This regulatory change will help promote the health supplement business in Thailand and ASEAN.
Indonesia
In Indonesia, the maximum limits for vitamins and minerals for use in health supplements were first updated by the Indonesian FDA (known as BPOM) with the issuance of BPOM Regulation No. 11 of 2020 concerning the Criteria and Procedure for Health Supplement Registration, which has since been revoked by BPOM Regulation No. 32 of 2022. There is currently a draft amendment to BPOM Regulation No. 32 of 2022 that has not yet been finalized. However, the draft amendment does not propose any changes to the current maximum limits for vitamins and minerals.
This shows how BPOM has been actively updating the regulatory framework on health supplements due to the large presence of health supplement products in Indonesia. Businesses in this sector therefore need to be sure of the latest requirements before registering their health supplement products in Indonesia.
Vietnam
In contrast to the changes seen in recent years in Indonesia and Thailand, Vietnam has not amended or drafted any amendments to domestic regulations related to the maximum levels of vitamins and minerals in health foods since 2014. The current maximum limits for vitamins and minerals in Vietnam are not matched to those in neighboring countries or to those in the ASEAN Agreement on a Regulatory Framework for Health Supplements. Nevertheless, Vietnam may consider revising the maximum limits at some point to comply with the ASEAN standards.
In Vietnam, health supplements are classified as food rather than medicine. Under Vietnam’s current regulations, the vitamin and mineral content in health supplements (calculated according to the manufacturer’s recommended daily dose) must not exceed the maximum intake threshold for vitamins and minerals prescribed in Circular 43/2014/TT-BYT.
If the maximum intake threshold is not specified, the related Codex regulations or those of relevant international organizations apply.
Breakdown
The following table summarizes the current maximum daily limits for vitamins and minerals in health supplements for adults in Thailand, Indonesia, and Vietnam. It also shows how the limits have changed compared to the previous regulations in each country.
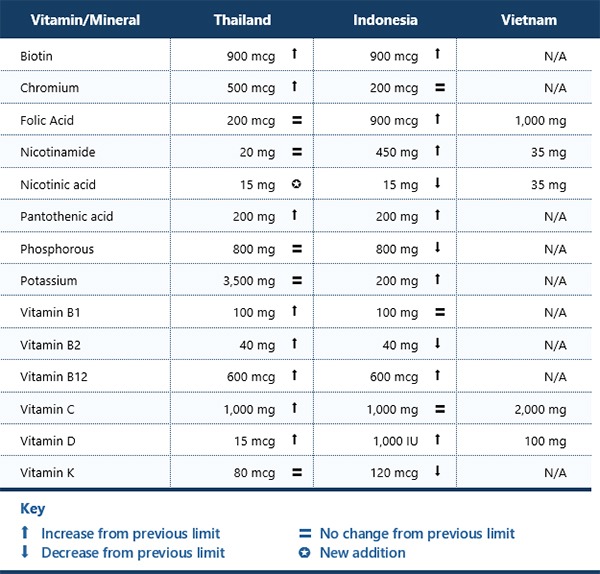
Where the limits are marked as not available, manufacturers may either follow the limitations under the ASEAN Agreement on a Regulatory Framework for Health Supplements or assume vitamins and mineral limits in health supplements based on generally accepted scientific data and taking into consideration, as appropriate, the varying degrees of sensitivity of different consumer groups.







